Partner with experts dedicated to you, your business, and your patients
Experience curating innovative solutions in healthcare
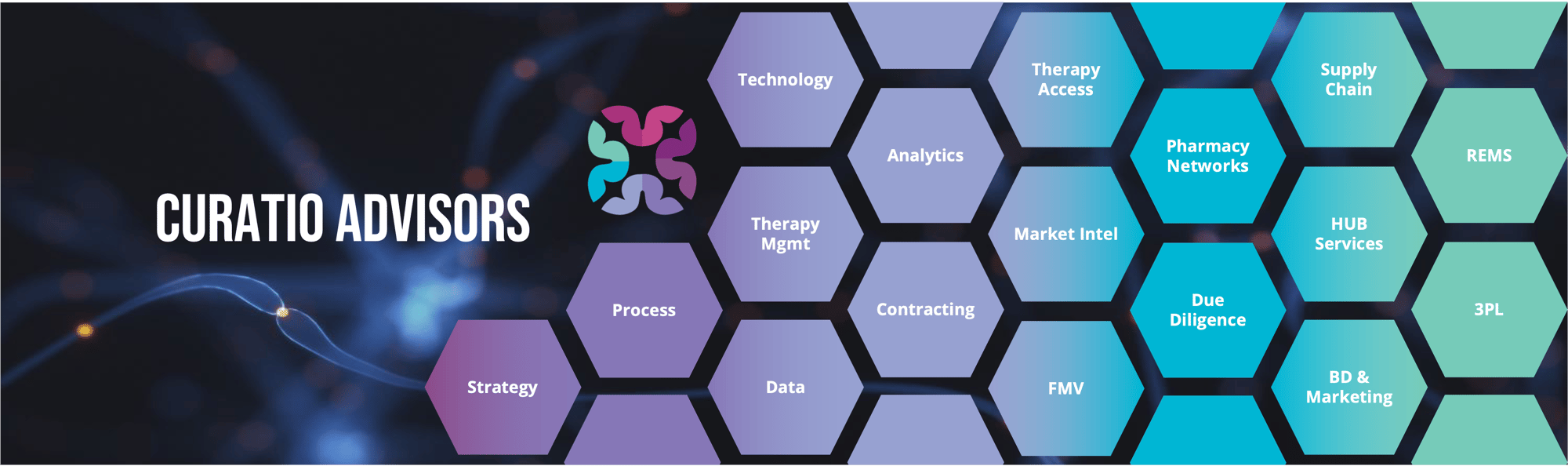
Curatio Advisors propels your business to the next level by helping you meet the needs of your patients. We help optimize patient access to care and improve outcomes with specialty drug therapies.
Entrepreneurs at heart, we are passionate about helping you accelerate the accomplishment of your goals. With deep experience in the specialty healthcare space, our team supports clients with pharmaceutical go-to-market product launches, specialty pharmacy operations, and private equity acquisitions.
DIFFERENTIATORS
High-Integrity, Unbiased Experts
As an independent advisory firm, we work solely with your team to curate innovative solutions. Our team holds no underlying agenda nor bias towards a particular technology, process, or vendor. We care deeply about your success, which is why we tailor solutions that fit your needs and optimize patient outcomes. We hold the utmost respect for your team, give it our all, and always go the extra mile to ensure success and satisfaction.

Services designed to best support you

Pharma Manufacturers
- Go-to-Market Drug Launch
- Channel Strategy
- Pharmacy Network Support
- Specialty Data & Analytics
Specialty & Infusion Pharmacies
- Pharmacy Operations Support
- Specialty Therapy Management
- Payor & Pharma Access
- Data & Reporting Solutions
Private Equity
- Market Assessments
- State Legislation & Compliance Support
- Due Diligence
- Reimbursement Audits
Pharma Services Providers
- Patient & Provider Journey Mapping
- Network Strategy
- Product Access & Reimbursement
- Specialty Data & Analytics
Clients over and beyond satisfied

"The go-to-market strategies provided by Curatio were instrumental in our product’s successful launch. They not only helped us navigate the complexities of market entry but also optimized our channel strategy, ensuring that we reached our target audience effectively. Their understanding of the industry and ability to tailor solutions to our specific needs was key to our success."

"Launching a new brand in a competitive market is always a challenge, but Curatio’s expertise made all the difference. Their comprehensive market analysis, coupled with their deep understanding of the pharmaceutical landscape, allowed us to make informed decisions that positioned our product for long-term success. Their support was critical in achieving our launch objectives."

"Partnering with this team transformed our pharmacy operations. Their expert guidance streamlined our processes, improved inventory management, and enhanced our overall efficiency. The results were evident almost immediately, with a noticeable improvement in patient satisfaction and workflow. Their dedication to our success made them an invaluable partner."

"Curatio Advisors supported us on a time-sensitive project to optimize our payor credentialing and contracting efforts. Their experience and support really made a difference for us at a critical juncture in our overall growth."

"Navigating payor contracts and securing pharma access has always been a challenge for us, but this team made it straightforward and efficient. Their deep understanding of the market and strategic approach ensured that we secured favorable contracts and reimbursement rates. They were proactive, responsive, and had our best interests in mind every step of the way."
CASE STUDIES
See our expertise in action
Discover how our advisory services have driven success for clients just like you. Explore our detailed case studies to see real-world examples of strategies, solutions, and results we deliver.

CONNECT WITH US
Have a quick question?
Need direct support, fast? Our team is available to answer questions specific to your unique needs. In just one business day, one of our experts can reach out to learn more about your needs.
